Kirsten Vinyeta, Frank K. Lake and Kari Norgaard
As Karuk people, we recognize other species in nature as part of an extended ecological family to whom we are related and have responsibilities. Leaf Hillman, Karuk Tishuniik ceremonial leader & DNR Director, describes this relationship and its associated responsibilities with reference to the Karuk Creation Story and the importance of World Renewal Ceremonies: “The rocks and the trees and the water and the air, the responsibility that I have, those are real relations. . . .We have not forgotten that we are related and that we have responsibility. And at the same time we give thanks to those other spirit people for helping to subsist us, and reminding them that we haven’t forgot that we owe them something too. So the renewal is renewing the bonds that exist.” This worldview has been referred to as “kincentricity” in the academic literature (Martinez 1995, Salmon 2000, Senos et al. 2006).

Acorns and huckleberries harvested in an area burned by prescribed fire. Photo: Stormy Staats, Klamath-Salmon Media Collaborative
Across the landscape, traditional food, fiber and medicine and especially water are vitally important for Karuk people. Hundreds of species from salmon and acorns, to tobacco and wild celery (kíshvuuf) provide materials necessary for cultural continuity, spiritual practice and the preservation of traditional knowledge systems. Some 150 culturally utilized plants are catalogued in “Plants and the People: The Ethnobotany of the Karuk Tribe” (Davis and Hendryx 1991), the Karuk herbarium catalogues over 100 species, but even more are recognized and used.
Traditional foods and medicines support physical and mental health in multiple ways (Alves and Rosa 2007). Cultivating, harvesting, processing, preserving and consuming Native food and medicine provide the framework for the Karuk eco-cultural socialization process and religious belief. Karuk traditional foods, especially salmon, are higher in protein, iron, omega-3 fatty acids, zinc and other minerals and lower in saturated fats than market foods (Norgaard 2005). Nutritional data show that traditional foods produce stronger hearts, blood and muscle tissue (Jackson 2005). The omega-3 fatty acids found in such abundance in salmon (and anadromous fish such as Pacific lamprey eels) have been linked with a number of significant health benefits including reduced risk of heart attacks, strokes, and Alzheimer’s disease, improved mental health and improved brain development in infants (Norgaard 2005). The often strenuous tasks of acquiring traditional food provides exercise that keeps people in good physical condition. Because hunting, gathering, fishing, storing and preparing food are an integral part of daily life and seasonal celebration, traditional food holds great cultural, religious and social meaning as well. These activities also serve as an important social “glue” by bringing people together to work, socialize and pass down values and information from one generation to the next (See Brown et al. 2011, Risling Baldy 2013).. Food is also central to some of the most serious social obligations for Karuk people – hospitality and caring for elders. Overall, the health benefits of eating traditional foods include better nutrient density, the availability of key essential nutrients, physical activity during harvesting, lower food costs, the prevention of chronic disease by consumption of more nutritious food, and “multiple socio-cultural values and traditions that contribute to mental health and cultural morale” (Kuhnlein and Chan 2000, p. 615, Cantrell 2001, Risling Baldy 2013, Fleishhacker et al. 2012).
This chapter examines the vulnerabilities to traditional foods and cultural use species in light of the increasing likelihood of high severity fire. Species of importance to Karuk people are impacted by other climate change related stressors that intersect with the vulnerabilities induced by the increasing instance of high severity fire. These other stressors, which include increased drought and temperatures, more variable weather, stronger storm systems, decreased snowpack, flooding, and increase in invasive species, are beyond the scope of this assessment, but may be mentioned here and are also discussed in Chapter One. As noted throughout this assessment, we take an intersectional approach to evaluating vulnerabilities resulting from high severity fire. This approach considers fire related vulnerabilities to Karuk foods and cultural use species in the context of past, present and future fire related management actions. Throughout this assessment we underscore that while high severity fire is a serious and immediate dimension of climate change, Karuk ancestral territory is fire dependent. Humans are ecosystem components and fire is medicine in Karuk culture (see also Wells 2014).
Humans as Ecosystem Components
Not only is climate change the result of human activity, humans are integral components of the mid-Klamath ecosystem in very specific ways. Karuk people have shaped the ecology, fire behavior and species composition in their ancestral territory through traditional management to enhance species of cultural importance (Anderson 2005, Halpern 2016, Lake 2007). The impact of past and present traditional management on ecosystems is of such magnitude that some argue, correctly, that American Indian land management should be considered part of the reference ecosystem when attempting to restore degraded landscapes (Senos et al. 2006). “Inhabiting one of the most complex geographical areas of North America, the Karok [Karuk] benefitted from great diversity in flora and fauna. The number of species support by the Klamath Mountain province is reported to be among the highest of any comparably sized region on the [North America] continent” (AITS 1982: 143).

Cultural burning at Tishaniik Ceremonial Grounds. Photo: Stormy Staats, KSMC
Karuk resource management practices, including fire use, increased pyro-diversity of existing ecological communities. “A review of earlier ethnographic work and more recent oral history interviews of tribal elders conducted by the Karuk Tribe and myself [Lake] suggest that tribal TEK encompasses a core area of knowledge about discrete fire events that contribute to landscape fire regimes. Tribal knowledge of fire ecology is closely coupled with subsistence economies, ceremonial practices, and individual or family adaptive strategies. Tribal TEK may also be able to describe how climate and weather influence fire behavior, from the yearly to decadal scale, with generalized understanding of century-scale climate and fire regime changes” (Lake 2013:4).
Karuk fire management is specifically linked to biodiversity (Lake 2013). Anderson (2005) highlights many uses of Karuk cultural burning, including as a means to reduce forest diseases and pests, increase seed and grain production, reduce the fuel load in forests, and enhance the quantity and quality of, and access to food and cultural use species such as oaks, hazel, beargrass, and native tobacco. Karuk fire regimes generate pyrodiversity on the landscape by extending the season of burn and shortening fire return intervals (Lake 2013, Martin and Sapsis 1992). The multitude of foods, materials and other products that come from Karuk environments are in turn evidence of the profound diversity of fire regimes that are required to maintain relationships with hundreds of animal, plant, and mushroom species (Anderson 2005, Lake 2007 & 2013, Anderson and Lake 2013). “For example, drier years have potentially greater fire spread and less resource productivity and required tribal groups to modify fire use and adapt foraging strategies. Tribal seasonal travel and resource strategies were likely linked to differing fire patterns across a range of similar vegetation types, but with each type having been burned at different frequencies. Thus developed a staggering of seral stages of similar vegetation communities across the landscape, differing by time since burn and by severity of burn. A landscape with burns in different years with mixed severities provided greater diversity of seral stages among vegetation types that facilitated tribal acquisition of valued resources” (Lake 2013:12-13)
The vulnerabilities faced by species of importance to Karuk people in the context of high severity wildfire do not occur in a vacuum. These vulnerabilities must be understood in the context of existing species susceptibilities (e.g. threatened and endangered status, mobility, range limitations), as well as the past, present and future management actions of Tribal and non-Tribal land managers. Not only does high severity wildfire hold the potential to negatively affect some species more than others for biological reasons, species that are already at risk or which have more difficulty in adapting will be at greater risk in the event of frequent large-scale high severity fires (Dale et al. 2001). Furthermore, past management actions from logging, road building or fire suppression interact with fire events to influence the level of vulnerability, as do management actions taking during a fire and those that may follow in the long term (Odion et al. 2004). Again, we consider the intersectional dimension of vulnerability to high severity fires in the context of past, present and future management actions.
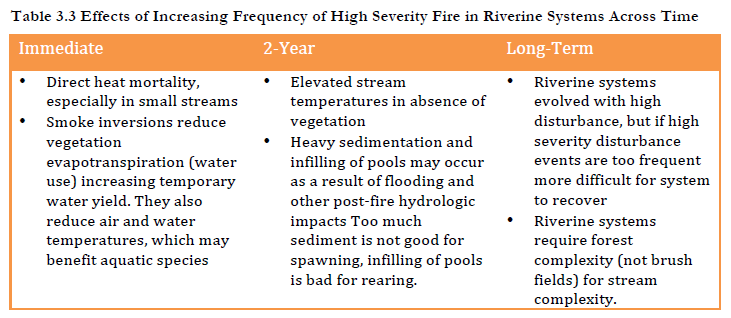
Since 1910, the activities of the U.S. Forest Service have shaped the ecosystems of the region in different ways. Fire suppression has been a dominant human influence, as have logging, road building, and the replacement of complex forest stands with even age, single species conifer “plantations” (Odion et al. 2010). Logging slash left on the forest floor and fire suppression dramatically increase the risk of high severity fires (Taylor and Skinner 2003). When high severity wildfires occur impacts to ecosystems and species of importance vary dramatically. Because the mid-Klamath region is fire adapted, even high severity fires have many positive dimensions for particular species and in particular time frames. For example, hydrological erosional processes contributing post fire sediment plumes to lower gradient creeks and rivers may smother salmon eggs or reduce fish habitat suitability in the short term if they come at the wrong time of year, but also bring needed woody material and replenish substrate (sand, gravel, rocks) which form longer-term habitat complexity (Wondzell and King 2003). The exact relationships between fire events and species impacts are sometimes debated. In light of the changing patterns of fire behavior, impacts of repeated high severity fire are often unknown. On the whole however, it is clear that while Karuk ancestral territory is adapted to repeated lower intensity mixed severity fires (Perry et al. 2011), the increased frequency of high severity fires creates serious vulnerabilities to the mid-Klamath ecosystem and particular species of importance to Karuk people. Impacts of high severity fire are complex and vary across space and time. We evaluate vulnerabilities at three temporal scales: those that occur during fire events, those occurring in the immediate aftermath of fires and long term vulnerabilities.
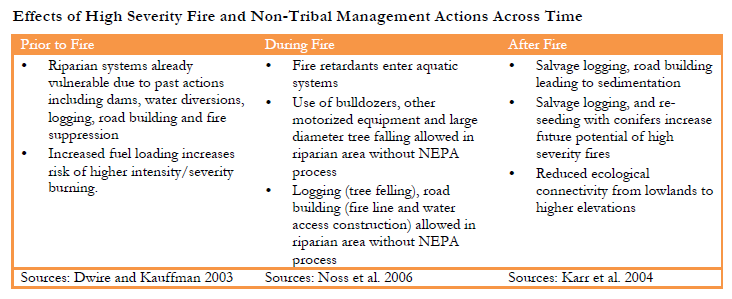
When high severity fires occur, they are the subject of additional management actions, usually some form of fire suppression (which has various degrees of success). Activities from back burning to the use of fire retardants are often carried out by agencies who are unaware of the intricate economic, cultural and spiritual relationships Karuk people have with species such as tanoak and madrone trees in the forest, or lamprey in the rivers. Fire suppression actions all too frequently cause further vulnerabilities to Karuk traditional foods, fibers and medicines. And long after fires have ceased to burn, management actions such as re-seeding, sediment control, road building and salvage logging cause further, often long term damage (Karr et al. 2004, Noss et al. 2006). Such activities create lasting impacts on the landscape by bringing in new species (i.e. invasives) that come into direct competition with culturally important Karuk species, increasing the future likelihood of high severity fires (Brooks et al. 2004), increasing sedimentation, and causing vegetation assemblage shifts. Understanding the climate-induced vulnerabilities in relation to high severity fire faced by particular species therefore requires an interdisciplinary multi methods approach that takes into consideration not only biological factors and fire science, but also traditional ecological knowledge and an understanding of the socio-political dimensions of land management in Karuk ancestral territory.
Recognizing that fire influences both individual species and landscape structure, we consider the impacts of high intensity fire on traditional foods, fibers and medicines first with a general discussion of six habitat zones, followed by single page species profiles that highlight how a given species is affected by cultural burning, versus how it is affected by high severity fire, and finally how fire related federal management decisions affect that species’ vulnerability. We use a culture-centric perspective on vegetation zones centered on the cultural keystone species of Tanoaks and Chinquapin (see Garibaldi and Turner 2004 for other species). We consider Riverine and Riparian species, Low Elevation Forest (defined as the Tanoak band), Grasslands, Middle Elevation Forest (defined as the Chinquapin band), High Elevation Forest (defined as above Chinquapin but below montane zone), Wet Meadow and High Country (montane and subalpine). This culture-centric zone model corresponds to the ecological model developed by Briles et al. (2005), but links vegetation to cultural keystone places across the landscape (Cuerrier et al. 2015).
For each habitat zone we provide a general discussion of the influences of fire in the ecology of each zone, describe potential threats in the face of increased fire severity and frequency, and discuss how the management actions of federal land managers intersect with the vulnerabilities engendered by high severity fire in the face of climate change. Where possible, these discussions are supplemented by tables to aid in organizational clarity. Information compiled in this chapter reflects a combination of Karuk traditional ecological knowledge and western science. These different habitat zones face distinct threats in light of increasing high severity fires for particular areas or the larger landscape. Some zones will have more species emphasized than others, yet while we use the zone approach to highlight the relationships between species in close proximity, it is important to understand that the zones also matter for their connections to one another. For example, wet meadows provide water storage that minimizes flooding in lower elevations, while low elevation tan oak is critical winter foraging habitat for elk who are in turn needed to sustain wolf populations. Where less information is presented or fewer species discussed, it does not mean that the zone is of lesser importance. For example, lamprey are a key food source, but have been less emphasized by western scientists, limiting information available for this profile Peterson Lewis 2009, Miller 2012). . There are relatively more profiles included for riparian and low elevation habitat zones where many species are used directly. Yet the high country habitat zone is critical for its influence on hydrological dynamics in lower elevations. The grassland zone was historically significant for a number of important species including elk, camas, brodiea and medicinal forbs. Today a majority of grasslands have disappeared due to lack of burning (see Skinner 2005), thereby impacting the depth and abundance of traditional ecological knowledge related to grassland habitats (Lake 2013). Restoring fire processes and function is in part about restoring the human responsibility to these species.
Following discussion of each habitat zone we provide an in depth profile for particular species occurring in that zone. Karuk people utilize hundreds of plants and animals for food, fiber and medicine; the species profiled here reflect only a small portion of all those that are culturally and ecologically vital, and were chosen to represent a range of elevation bands, flowering times and dimensions of vulnerability. Many of the profiled species are regalia species that are vital to traditional ceremonies. Many would be considered cultural keystone species (Garibaldi and Turner 2004). To the extent possible, each profile compiles information regarding the influence of cultural burning on the species, as well as the vulnerabilities resulting from the increasing frequency of high severity fire at three scales (during fires, in the immediate aftermath and long term). Many species occur across multiple zones, or move across zones seasonally. In such cases profiles are included within the zone for which habitat is most critically limited.
What we have outlined below is not intended to be an exhaustive, definitive list, but is instead a starting point meant to illustrate inter-species, inter-habitat, and human/fire relationships—relationships which must be understood in order to formulate a course of action. Some or all of the species listed below may be considered focal, or indicator species in future management practices, or perhaps other indicators will emerge under adaptive management principles. Regardless, the foundational premises and values underlying the ecological management principles and concerns outlined in these habitat and species profiles are of enduring relevance.
It is also important to note that while high severity fire, particularly when occurring in rapid succession, is considered a threat to the eco-cultural health of the region, it also inevitably brings with it some of the same ecological benefits that result from Karuk cultural burning. It is not fire, or even the occasional high severity fire per se, that is the problem, but the repeated occurrence of high severity fire (resulting from climate change) combined with land management practices that not only promote a cycle of increasingly frequent high severity fires, but that also have negative impacts on habitats and species of cultural importance. In this chapter, we tend to highlight the negative implications of high severity fire, while focusing on the benefits of cultural burning practices. However, there are instances in which we also note benefits that may result from high severity fire. It is possible for high severity fire to be detrimental for some habitats and species, while simultaneously benefitting other habitats or species. It is also possible for fire to benefit a species or habitat in one way, while being detrimental in a different way.
Riverine Vulnerabilities
Karuk ancestral territory encompasses several hundred miles of riverine habitat along the middle portion of the Klamath river, the lower portion of the mainstem Salmon River, and many key tributaries. Species from riverine systems hold significant cultural and spiritual significance and provide over fifty percent of the calories and protein of traditional Karuk diets (Kroeber and Barrett 1960, Chartkoff and Chartkoff 1975), Salmon (coho (achvuun), spring (ishyat) and fall chinook (áama)) and other riverine species including green sturgeon (ishxíkihar), lamprey (Klamath and Pacific (akraah)), steelhead trout (sáap), river otter (pay saruk/amváamvaan), and freshwater mussels (axthah) are important for food, culture and ceremonies.
Riverine systems are especially at risk in the face of changing patterns of precipitation, increasing temperatures and decreasing winter snow pack resulting from the changing climate (Mote et al. 2003, Barr et al. 2010). Beamish and Bouillon 1993). At the same time, local conditions within riverine and forest systems play a significant role in habitat quality and species vulnerability projections (Isaac et al. 2010). Wildfire from both human and natural ignition has been an integral component of the riverine systems in the mid-Klamath region. Fires are particularly important for shaping the local quality of riverine habitats in the face of climate trends (Hamlet 2011).
Relationships between fire and riverine habitats are complex and vary by species, fire intensity, fire severity and time frame (Isaac et al. 2010, Rieman et al. 2003)). While Karuk use of fire is often noted in relation to forest systems, cultural burning is also critical for riparian and riverine habitats (Lake 2007). Many riverine species of importance, including salmon, require complex habitats with large woody debris. Fires bring sediments and large woody debris into stream systems critical for both stream productivity and habitat complexity (Arkle and Piliod 2009 and 2010). Low intensity fires are important for stream flows as they clear out brush that uptakes water, while high intensity fires are needed to generate debris inputs Biswell 1989). High intensity fires may have negative short term impacts on riverine species, but are nonetheless critically important in the long run, since high intensity fires in particular provide additions of gravel and logs, and generate the canopy opening that form a habitat mosaic of more and less productive stream habitats (Arkle and Piliod 2009 and 2010, Davis 2016).
Fire influences on riverine systems also vary across time. During fire events reduction in vegetative evapotranspiration often increases stream flow (Biswell 1989). Larger regional or multiple local fires burning during atmospheric high pressure, coupled with river canyon smoke inversions (Robock 1988 and 1991)can be beneficial to spring salmon (see Strange 2010 for cold fronts/clouds similar to the effects of smoke). The smoke may cool river temperatures during critical warm periods, creating better conditions for various fish species and age classes, including fall migration and spawning of Chinook salmon (Lake and Tripp, personal communication, David and Lake 2016). On the other hand, high intensity fires may cause elevated stream temperatures and direct mortality of fish and other species, especially in smaller systems (Hitt 2003).
In the immediate aftermath of high intensity fires (e.g. roughly 24 months) the absence of riparian vegetation that would otherwise provide shade may elevate stream temperatures (Dwire and Kauffman 2003, Isaak et al. 2010, Pettit and Naiman 2007). Post fire debris flows may reduce fish numbers and negatively alter fish habitats (Burton 2005). Yet many of these events are short term, while longer-term impacts of fires include increases in stream productivity and beneficial changes in fish diet (Malison and Baxter 2008 and 2010). Work by Flitcrof et al. (2016) on fire and Spring Chinook habitat quality in stream networks, and work by Isaak et al. (2016, 2010) on stream temperatures examine the complex conditions in which a beneficial ‘pulse’ disturbance turns into a possibly less beneficial ‘press’ disturbance. In general, unless species are vulnerable or populations are highly fragmented, populations usually rebound successfully (Rieman et al. 2003).
Over the long run regular fire on the landscape reduces the amount of brushy vegetation (i.e. shrubs and younger vigorously growing trees) withdrawing water from creeks, which affects steam flow and thereby stream temperatures. Post-fire floods can remove fine sediments and provide much needed gravel, cobble, woody debris, and nutrients. Longitudinal studies by Burton (2005) found that fire restores stream habitats, resulting in higher fish productivities than were present pre-fire. High intensity fire in particular leads to larger debris flows and tree mortality (Gresswell 1999). The disturbance mosaic resulting from high intensity fire is like a patchwork quilt of habitats across stages of recovery. This mixed severity burned landscape releases moderated amounts of sediment and water streams through springs and seeps, improving larval habitat for species such as Pacific and Klamath Lamprey. These effects of cultural burning on the riverine system are summarized in Table 3.2 below.

Vulnerabilities Resulting from Increased Frequency of High Severity Fire
While both high and low intensity wildfires are important to the riverine systems of the mid-Klamath, less is understood about how increase in the frequency of high severity wildfire will impact riverine systems (Flitcroft et al. 2016). Extensive areas of large high severity fires burning across streams, creeks, and rivers can have negative impacts on stream temperatures and can generate fish mortality during fire events, especially in smaller systems. Frequent high severity wildfires can eliminate foliage needed for slope stabilization, leading in turn to more frequent and larger landslides and debris flows, which can degrade fish habitat in the short term by increasing temperatures and more. Aquatic species are particularly sensitive to such habitat disruptions given their dependence on clear, cold water and their limited ability to relocate (Fagan 2002).
Perhaps most importantly, increases in the frequency of high severity fires cause habitat shifts that reduce forested habitats that develop under lower frequency or mixed severity fire regimes and promote the presence of shrub and herbaceous assemblages that thrive under higher frequency fire conditions. Essentially the surrounding forest could shift to an early seral stage that has a higher risk to (re-) burns again and again, unable to return to mature forest condition (burning seed sources before re-establishment of trees). Such a shift of the forests surrounding riverine systems to brush fields would have very serious consequences for river temperatures, species composition and the structure of riverine habitats. From a physical standpoint, the complex forest structure that results from repeated mixed severity fire leads in turn to complexity of riverine habitats (Bisson et al. 2003, Perry et al. 2011). Indeed the complexity of stream habitats is directly linked to habitat complexity in the surrounding forest landscape (Bisson et al. 2003, Flitcroft et al. 2016). This is true in part because fires are a form of disturbance that shapes physical characteristics of upslope forests and riparian environments, including opening canopy and enhancing regeneration (Hessburg et al. 2005 and 2007, Perry et al. 2011, Swanson et al. 2011). In the event that forests were entirely or partially converted to brush such forested complexity would no longer exist. Water temperatures would elevate as flows dropped and less shading occurred from vegetation. Warming stream temperatures in turn enhance suitability for non-native fishes (Durham et al. 2003).
Vulnerabilities from High Severity Fire Exacerbated by Non-Tribal Management Actions The vulnerabilities riverine systems face in light of the increasing frequency of high severity fire must be understood in the context of actions taken by other public and private land managers prior to, during and after high severity fires. Riverine systems are already threatened in the mid-Klamath area due to existing pre-fire management actions that include a combination of dams, water diversions, water quality impairments from agricultural inputs, logging activity, fire suppression and failing roads. In particular, fire suppression has removed the many benefits of fire to rivers and streams including limiting the natural ‘disturbance mosaic,’ thereby limiting beneficial wood and gravel debris, reducing stream flows and the additional water inputs to streams and rivers after fires (Gresswell 1999, Noss et al. 2006). This disturbance mosaic provides a high diversity of habitat types and gives different species options. Fire suppression has had a direct impact on specific species including salmon and lamprey as well. A Karuk tribal community member quoted in Peterson (2006) notes: “Ammocoetes that are in the fine mouth of these tributaries aren’t getting the kind of hydrograph with the quantity and quality of water that they had historically” (p. 70). Fire suppression has also created vulnerabilities in the riverine system by leading to more frequent and extensive high severity fires (Taylor and Skinner 2003), and the associated vulnerabilities descried above.
During high severity fire events the management actions of federal and state agencies, especially the US Forest Service, may exacerbate vulnerabilities to the riverine system. Detrimental fire suppression activities such as: Fire retardants spill into springs, creeks, rivers and lakes. Road building (opening decommissioned or closed roads), creation of fire lines, safety zones or escape routes(dozer/equipment clearings) in vicinity of riverine systems, excessive felling of mature/old growth trees in riparian areas. After fires, Forest Service management actions including salvage logging sales, limited road building and replanting with conifers which affect riverine health and future high severity fire potential (Dwire and Kauffman 2003, Noss et al. 2006, Stephens and Ruth 2005). These impacts are detailed in Table 3.4 below.

In order to further illustrate the complex of vulnerabilities high severity fire poses for Karuk species of importance in riverine systems we provide species profiles for spring chinook salmon (áama) and Pacific lamprey (akraah).
Salmon / Ishyá’at / Oncorhynchus tshawytscha
Pacific Lamprey (Eel) / Akraah / Lampetra tridentata
Riparian Vulnerabilities
Riparian areas are key sites for many food, fiber and medicinal species of importance to Karuk people. Focal species include (but are not limited to) Pacific giant salamander (puff puff), aquatic garter snake (asápsuun), beaver (sahpihnîich), mink (Xanchun’ámvaanich), cedar waxwing (akravsiip), yellow-breasted chat, big leaf maple (saan), mock orange (xávish), huckleberry (púrith), horsetail (chimchîikar), and multiple willow and fern species including woodwardia (tip-tip) and five-finger/maiden hair ferns. Some of these species are also discussed under the section on vulnerabilities to riverine systems and wet meadows. The health of riparian areas is also important for the functioning of riverine and forest systems and the habitat quality for the species within.
Fire is an important component of riparian systems in the mid Klamath and cultural burning in the surrounding forest has key impacts on riparian habitat quality (Lake 2013). The Karuk Draft Eco-Cultural Management Plan outlines how riparian species composition, vegetation structure and hydrology are shaped by the use of fire: “Certain trees and shrubs utilize water more than others, fire affects this relationship (Fites et al. 2006). The distribution of forests, shrubs, and grasslands, affects the process of infiltration from precipitation and resultant levels of evaporation with how those plants utilized water (DeBano et al. 1998). The balance of water in and water out, leading to the amount of moisture in the soil and the quantity and quality of springs is influenced by fire (Biswell 1999:157)” (Karuk DNR 2010). Karuk fisheries biologist Kenneth Brink describes this relationship:
We did our fire management, which enabled to put more water into the tributaries, say like on a drought year, you take all your understory out, like all these blackberries and stuff would never be here. These alders would not be all big. There might be one or two big ones making a shade instead on all these little suckers. With burning in the past, you didn’t see the alder or the willow trees, you had willow brush. All this foliage takes up a lot of water.
Lower intensity mixed severity cultural burning further affects dynamics within riparian systems by releasing nutrients to the soil. Many specific riparian species of importance to Karuk people also benefit from low intensity cultural burning. For example, the Cedar waxwing (a regalia species) needs fire to maintain an open understory along riparian areas for breeding habitat. The yellow-breasted chat is is dependent on willows within cottonwood galleries along riparian sandbars at lower elevations (Lake, pers. comm. Bagne and Purcell 2011). In other cases, frequent fire is necessary to produce specific qualities in plants needed for cultural uses (e.g. straight shoots for basketry Anderson 1999). Beavers benefit from fire in that fire promotes plant species critical for the beaver diet, such as willow, and also affects riparian structure and hydrology in ways that benefit beaver habitat, for example the input of woody debris resulting from cultural burning provides material for dam construction. Beaver are in turn an important component of riparian and riverine systems for the benefits they bring to other species. For example, beaver dams benefit juvenile coho salmon by modifying the velocity, cover and depth of low gradient cold-water refugia.
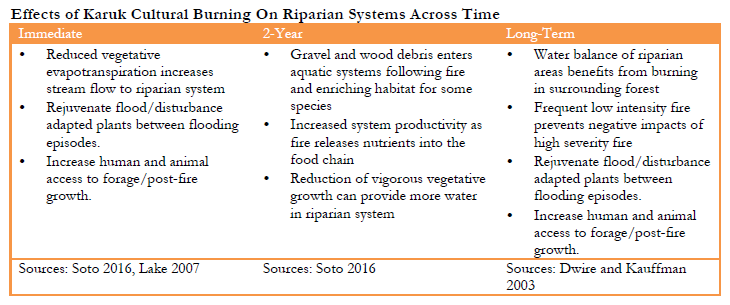
Vulnerabilities Resulting from Increasing Frequency of High Severity Fire
Moist conditions in riparian areas provide a relative degree of protection from direct mortality as compared to species in forests or grasslands, yet with high severity fires, direct mortality during fires may still occur (Dwire and Kauffman 2003). Large debris flows that occur following high severity fires may also cause direct species mortality. Serious threats to riparian areas may result if increases in the frequency of high severity fires cause habitat shifts that reduce forested habitats which develop under lower frequency or mixed severity fire regimes and promote the presence of shrub and herbaceous assemblages which thrive under higher frequency fire conditions. Such a shift of surrounding forests would have potentially serious consequences for riparian species composition, hydrology and structure. Riparian areas in the context of their landscape setting/position is also important to consider, as wide flood plain willow-cotton wood forest compared to mountain alder-maple/conifer dominated topographically steep areas (see Pettit and Naiman 2007)
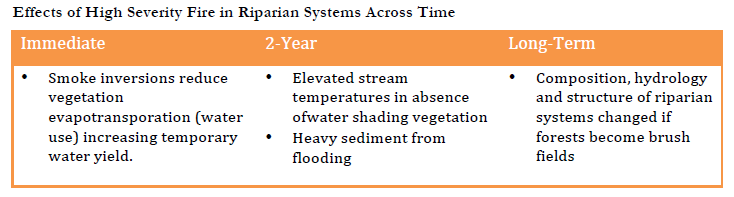
Vulnerabilities Exacerbated by Non-Tribal Management Actions
The vulnerabilities riparian systems face in light of the increasing frequency of high severity fire must be understood in the context of actions taken by other agencies prior to, during and after high severity fires (Dwire and Kauffman 2003, Bisson et al. 2003). Riparian systems are already threatened in the mid-Klamath area by logging, roads and fire suppression. Fire suppression intersects with climate change to increase the likelihood of more frequent high severity fires (McKenzie et al. 2004). Sediment from past logging and road building activities, as well as poorly maintained roads has increased stream temperatures in riparian areas. Inputs of fine sediments, alter stream hydrology, may eliminate salmon spawning habitat by filling it in with sand, and can smother salmon redds, suffocating eggs (Gresswell 1999, Rieman et al. 2003).
During high severity fire events riparian systems face additional threats from fire fighting activities (Noss et al. 2006). Road building and the use of bulldozers and the cutting of trees are allowed to occur without NEPA process as an “emergency exemption for an act of nature” during high severity fire events. While the use of fire retardants is not permitted in riparian areas, accidents occur on a regular basis. Lastly, the longer-term aftermath of high severity fires is increasingly characterized by proposals for salvage logging operations (Karr et al. 2004, Noss et al. 2006). Here too normal procedures set in place for water quality protections have been waived, reducing protections on riparian systems.
To illustrate the threats high severity fire poses for Karuk species of importance in riparian habitats we provide species profiles for four riparian species of cultural importance: Pacific giant salamander (púfpuuf), aquatic garter snake (asápsuun), beaver (sahpihnîich), and yellow-breasted chat.
Pacific Giant Salamander / Púfpuuf / Dicamptodon tenebrosus
Aquatic Garter Snake / Asápsuun / Thamnophis atratus
Beaver / Sahpihnîich / Castor canadensis
Yellow-Breasted Chat / Icteria virens
Low Elevation Forest: Tanoak Zone Vulnerabilities
Lower elevation forest habitat (roughly 500-3,000’) within Karuk ancestral territory and homelands is characterized by the presence of tankoak trees (xunyê’ep) as cultural keystone species. Not only have tanoak acorns traditionally constituted a high percentage of the calories and protein of Karuk diets (Norgaard 2005), tanoaks are culturally and spiritually significant. Low elevation tanoak forests contain an abundance of species of direct importance for Karuk food, fiber and medicine. These include fungi: tanoak mushrooms (xáyvi’ish, commonly known as matustake), black trumpets, hedge hogs, fang, and other mushrooms (see Anderson and Lake 2013), herbs:princess pine (xunyêepsurukhitihan), Oregon grape, Yerba Buena-mint, shrubs: huckleberry (púrith), California hazel, mock orange, service berry, ceanothus, ocean spray; trees-, black oak, port orford cedar, California bay (pahiip), canyon live oak (xanputtin), madrone (kusrippan), ponderosa pine (ishvirip), white oak (axveep); animals: pileated woodpecker (iktakatákkahe’en), black-tailed deer, fisher, coyote (pihneefich), black bear (virussur), ringtailed cat (tapukpuukanach), gray squirrel (axruuh), and the winter range of elk (íshyu’ux).In addition to the direct importance of this habitat zone to particular species, the stand dynamics and fire regimes of low elevation tanoak forests significantly shape riparian and riverine health.
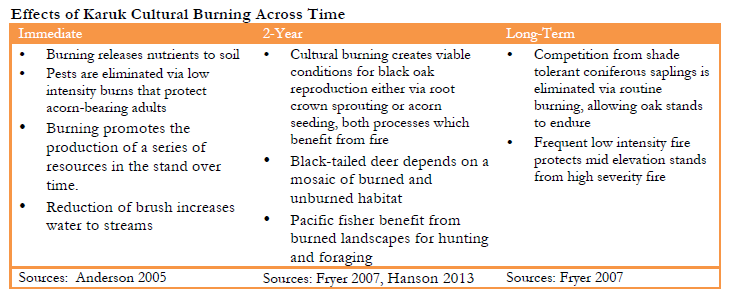
Both the composition and overall stand structure of low elevation tanoak forests is a direct result of their long term intensive management by Karuk people through the use of fire (Anderson 2005, Bowcutt 2013, Halpern 2016, Karuk DNR 2010, Martinez 1995, Lake 2007 and 2013, McCarthy 1993). Frequent fires limit the encroachment of competing shrubs and conifer species (Turner et al. 2011, Perry et al. 2011). Low intensity fire favors oaks over conifer species in part because oaks can re-sprout and thereby reestablish after fires (Hosten et al. 2006, Cocking et al. 2012. By contrast, competing species such as cedar, fir and pines reproduce with seedlings that will burn up (Plumb 1981). The open structure of these forests is important for many other species including madrone, white and black oak, pileated woodpeckers and elk. Indeed, as is true for many other California Indians, the majority of species Karuk people use thrive in either open forest conditions or full sun (Anderson 2005, p. 152). At the larger scale, traditional burning at multiple fire frequencies promotes a mosaic of vegetation types in different stages of response to fires. This diversity of food species in multiple phases across the landscape supports food security for Karuk people (Busum 2006, Kimmerer and Lake 2001, Lake 2013). The frequent use of low intensity fire is especially important for overall stand structure given that tanoak trees are quite vulnerable to high severity fire (Bowcutt 201, McDonald and Vaughn 2007). Stands that are clear of underbrush can be burned again with lower risk of damaging mature oaks. Fire exclusion has reduced the diversity of resource patches in the landscape causing what had been distinct bands of groupings that were burned in their own cycles to blend together (Lake pers comm, re ecotones of oak-dominated habitats with more forested or grasslands, see Lake 2013).
Frequent burning also affects the water dynamics within low elevation forests. These relationships between brush, conifer reduction and groundwater are also of critical importance to species in the riverine and riparian zone. Low intensity fire breaks down organic matter, releasing nutrients to the soils where they become available for plant use. Tanoak mushrooms also have a symbiotic relationship with tanoak trees and other species including huckleberry (see Anderson and Lake 2013 for Karuk tanoak forest uses) and make nutrients available across species through their mycorrhizae.
In addition to the importance of frequent low intensity fire for the overall forest structure, frequent use of fire benefits many species in this zone directly (Skinner et al. 2006). McCarthy (1993) writes “In addition to promoting a variable distribution of oaks in the woodland community, the use of fire may positively affect individual trees and their yield” and quotes Scheneck and Gifford (1952) in noting that “Karuk women reported that the trees are better if they are scorched by fire each year. This kills disease and pests” (p. 221). Burning around trees enhances their health and the quality of acorns by reducing populations of filbert weevil and filbert worms (Halpern 2016).. Elk who especially use low elevation tanoak forest in winter, have been described as a “fire-follower” as they benefit from the effects of fire on their food sources (Patton and Gordon 1995). Elk in particular prefer a mosaic landscape that combines open areas for foraging, and forested areas for cover (Long et al. 2008). Pileated woodpecker benefit from the presence of intermediate scales of mixed severity burn patches across the landscape that foster nesting, roosting, and foraging habitats (Bull et al. 2007, Hartwig et al. 2004). Regular burning benefits tanoak mushrooms by minimizing forest duff. This protects the mushroom’s mycelium that can otherwise encroach into the duff where it becomes vulnerable to fire (Anderson and Lake 2013). Burning of the mycelium affects not only the mushroom itself, but the nutrient transfer system between tanoak trees and other species including huckleberry (Lake pers. comm., Anderson and Lake 2013). However, for the tanoak mushrooms, cultural burning is a more secondary benefit in that burning benefits the other species with whom the mushroom is a functional cohort. For example, once tanoaks reach 16-18 inches in diameter the production of mushrooms around them increases (Lake 2007).

Vulnerabilities Resulting from Increasing Frequency of High Severity Fire
While low intensity fires are an integral component of the tanoak forest zone, the increasing frequency of high severity fire in light of climate change poses serious vulnerabilities for this forest type (Bowcutt 2013). High severity fire creates vulnerabilities for individual species and for stand dynamics as a whole. Harrington (1932) quotes “a Karuk woman” “the tanoak is not good when it is burned off, the tree dies. When they are burning, they are careful lest the trees burn (p. 65).” While tanoaks are able to re-sprout after low intensity fire, their thin bark makes them highly susceptible to damage or mortality from hot (higher temperatures and durations of heat) fires. During high intensity or high severity fires the thin bark of this species may heat up and burn quickly leading to damage and scarring of the inner cambium and in turn cause disease and heart rot (see McCarthy 1993 she also cites Plumb and Gomez 1983). Tanoak trees produce a thick smoke when burning which is reported to have negative human health impacts (Lake pers. comm.). High severity fire events may also cause direct mortality to tanoak mushrooms ifreproductive mycelial mats are damaged. High severity fires consume snags and logs used by pileated woodpeckers for nesting, rooting, and foraging, and reduces insect populations as well as nut and berry sources that are vital to the woodpecker diet.
Ultimately the greatest vulnerability from the increasing frequency of high severity fire would occur in the event that tanoak stands (e.g. tribal food orchards) become ceanothus brush fields rather than mixed hardwood woodlands. With repeated very hot fires the structural integrity of tanoak stands might be destroyed. Given that many of these species depends on moisture and cool temperatures provided by shade from larger trees there is concern that high severity fire over time could reset (shift to an alternate vegetation state) the entire system, leading to the replacement of these important food and cultural use species to brush and chaparral. Tanoak stumps may sprout back as a large number of shrubby sprouts that do not produce cover needed to support shade tolerant species such as huckleberry. Without understory these brush stands lack microsite diversity. Brush fields may have be grassy component that can also cause the stand to burn more frequently preventing the return of mature larger diameter, trunked trees. If entire tanoak stands are destroyed by high severity fire, the many other species with which they are interdependent including tanoak mushrooms would in turn also be unable to repopulate given their symbiotic relationships with the oaks.

Vulnerabilities Exacerbated by Non-Tribal Management Actions
Vulnerabilities to species in the tanoak forest zone in light of high severity fire are magnified by the actions of other agencies prior to, during and after high severity fire events, see Table 3.5 below. Low elevation tanoak forests within Karuk ancestral territoryhave been significantly impacted by the past 100 years of fire exclusion (Skinner et a 2006). Fire exclusion has led to a buildup of fuels and “dramatic increase in the likelihood of high severity fires (Taylor and Skinner 2003). During fire events tanoak stands may be subject to destruction through back burning and the building of fire lines. Fire lines cutting through tanoak stands may damage or destroy the tanoak’s mycelium net. In the immediate aftermath of high severity fires activities such as salvage logging and associated road building also impact tanoak stands (although the tanoaks themselves not the target species).

In order to further illustrate the complex of vulnerabilities high severity fire poses for Karuk species of importance in this elevation zone we provide species profiles for tanoak (xunyêep), tanoak mushrooms (xáyviish), elk (íshyuux), huckleberry (púrith), and pileated woodpecker (iktakatákaheen).
Tanoak / Xunyêep / Lithocarpus densiflorus
Tanoak Mushroom / Xáyviish / Tricholoma magnivelare
Roosevelt Elk / Íshyuux / Cervus occidentalis
Evergreen Huckleberry / Púrith /Vaccinium ovatum
Pileated Woodpecker / Iktakatákaheen / Hylatomus pileatus
Wolf / Ikxâavnamich / Canis lupus
Grassland Vulnerabilities
Grasslands also known as prairies historically occurred in mid to upper montane areas on ridges, in both large and small patches up to elevations of 3,280 feet, especially on shallow ultramafic soils (Anderson 2005, Skinner et al. 2006). Today a majority of the grasslands that once existed within Karuk ancestral territory have disappeared due to lack of fire (Skinner 1995, Lake 2013). Traditional. Traditional Karuk knowledge about grasslands has unfortunately also been lost. Yet place names contain references to species such as wild oats. Grasslands have been historically significant for many species of broad-leaved herbs, native annual and perennial grasses, insects, birds, mammals, reptiles, and amphibians (Swanson et al. 2014). Amongst these are important Karuk foods and cultural use species such as Elk, Iris and other grasses used for twine, and a group of geophyte plants known as “Indian potatoes” which include multiple members of the Lilly family, Blue Dicks, White Hyacin, Golden Lantern, Soap Root, Yellow Globe Lilly (Lanctot and Lake ND, Anderson 2005, Schenk and Gifford 1952). Anderson (2005) notes the importance of prairies and grasslands for Indian people across California and lists a number of species of importance in these areas:
“Among the important grass species in California’s coastal prairies were Idaho and red fescues (Festuca idahoensis and F. rubra), California oatgrass (Danthonia californica), and bent grass (Agrostis exarata). Characteristic broad-leaved species were Douglas iris (Iris douglasiana), California buttercup (Ranunculus californicus), and blue-eyedgrass (Sisyrinchium bellum). Other species were yampah (Perideridia kelloggii), goldfields ( Lasthenia spp.), and tidy-tips (Layia platyglossa). . .
Anderson goes on to note the significance of the diversity of these grassland habitats:
California’s coastal prairies provide a good, well-researched example of how native practices promoted vegetational heterogeneity and high biodiversity. According to the ecologist Mark Stromberg, “The coastal terrace prairies in California and Oregon are the most diverse grasslands in North America. If you count the number of species in a square meter of California’s coastal terrace prairie you average 22.6 species—more than the inland prairies of the Midwest, which have between 8 and 12 species” (pers. comm. 2001). (66)
Grasslands in particular require frequent burning to maintaining the open prairie structure. Burning prevents conifer encroachment and enhance conditions for key food species, including reducing competition for geophytes such as brodiaea and camas and increasing soil productivity by releasing nutrients. (Anderson 2005, Stone 1951). Fires enhance the production of bulblets of many of the species known as Indian potatoes. Until about 1850, grasslands were so extensive they covered nearly one-fifth of California (Anderson 2005, 28). Anderson notes “The coastal prairies were burned to produce more food, reduce brush or trees, produce new grass for thatch, drive grasshoppers, enhance cordage materials, and increase forage for ungulates. Indian-set fires modified the grassland to fire-resistant species and expanded the grassland vegetation type (2005, 167). Local traditional knowledge of geophytes was emphasized by Harrington: “But they (Karuk People) knew indeed that where they dig cacomites (bulbs/corms) all the time, with their digging sticks many of them grow up, the following year many grow up where they dig them. They claim that by digging Indian potatoes, more grow up the next year again. There are tiny ones growing under the ground, close to the Indian potatoes” (Harrington 1932:73).
Vulnerabilities Resulting from Increasing Frequency of High Severity Fire
While burning is essential for grassland habitats, high severity fire has the potential to scorch soils. Former prairie-grasslands that have been encroached and colonized by trees develop higher carbon-rich biomass that can burn with detrimental effects to soil productivity. Frequent lower to mixed-severity fires that gradually reduce fuel loading can buffer negative impacts to soil (Neary et al. 1999). The anticipated effects of climate change on prairie ecosystems involved synergistic distrubances from management and ecological processes (Bachelet et al. 2011).
Vulnerabilities Exacerbated by Non-Tribal Management Actions
Probably the main intersecting vulnerability to grasslands comes from their severely reduced range due to fire exclusion (Skinner 1995). Grasslands are a threatened ecosystem type due to fire exclusion. Anderson writes “in the absence of fire, grassland ecosystems become choked with detritus, and productivity and reproduction fall drastically. Other studies show that grain production in most native perennial grasses dwindles in the absence of some kind of intermediate disturbance, such as herbivory, fire, or flooding. Furthermore, many of the herbaceous plants with edible seeds have high light requirements and grow only in open grasslands or light gaps in forests and shrublands” (2005, 178-179). To illustrate the vulnerabilities high severity fire poses for Karuk species of importance in grasslands we provide a species profile for Indian potato (tayiith).
Indian Potato / Tayiith / Brodiaea coronaria
Middle Elevation Forest: Chinquapin Band Vulnerabilities
The middle elevation chinquapin forest habitat (roughly 2,500 to 3,500’) is comprised of a number of culturally critical species that contribute important traditional foods and regalia. These include chinquapin (sunyíthih), black oak (xánthiip), saddler oak (yávish), white oak (axvê’ep), live oak (xanpúttip), yew (xupári’ish), port orford cedar (check elevation) (kúpri’ip), pacific fisher (tatkunuhpíithvar), and black tailed deer (púufich), prince’s pine, ponderosa pine (ishvirip), Douglas fir (itharip), hazel (surip/aththip), incense cedar (chuneexneeyaach), huckleberry (púrith), jeffrey pine (ishvirip), knobcone pine (ishvakippis) and porcupine (kaschiip).
As is the case with the lower elevation forest, the continued persistence of this forest type is highly dependent on fire and indigenous cultural burning (Anderson 2005, Cocking, Fryer 2007, Lake 2013, Lake and Long 2014, Long et al. 2016, Morgan and Sheriff 2012). The composition and structure of these middle elevation forests are fire adapted (Skinner et al. 2006). McCarthy (1993) writes that “Black oaks in particular would not have either their present distribution or their frequency with fire, and studies have shown that fire begun by natural causes (i.e. lighting) would not have occurred frequently enough to create that disturbance” (p. 220).
Middle elevation forests with black and other oaks, chinquapin, Douglas fir, hazel, and gooseberry would traditionally be burned as frequently as every 5-7 years (Lake pers. comm. based on Skinner unpublished studies, see also Pullen 1996 for Karuk burning).). Black oak acorns in particular are food for a variety of wildlife and the trees provide valuable pacific fisher denning habitat (North 2012, Long et al. 2016). Karuk management created a system of temporally staggered “resource patches” in the landscape. Burning promotes the production of a series of resources in the stand over time (Lake 2013). The first spring after fires generates a lush re-sprout of forbs and greens that in turn draw in deer and quail for hunting. As grasses become outcompeted over time, another set of foods and medicines becomes available; this next series of plants draws in a new set of associated species to the forest patch. Two years after the fall burning will be good for the production of basketry materials, after 3 years black cap raspberries will begin fruiting again, whereas bracken fern comes in after 7 or 8 years. At this point the acorns begin to get buggy and the burning cycle is ideally repeated (see Aubrey in Lake 2007 about cultural burning and food plots). In the absence of fire, however, the middle elevation chinquapin forest band is susceptible to encroachment by shade-tolerant conifers (Stuart and Salazar 2000).
Individual species in the chinquapin forest zone also benefit from the frequent occurrence of lower intensity fire. Karuk cultural burning sought to optimize berry production. For example the roughly ten year Karuk burning cycle keeps huckleberry into an optimal intermediate disturbance phase that maximizes production and cover (Lake, pers. comm.). In the absence of fire conifers compete with the oaks for resources. Conifers not only increase fuel loads within the stand, they reduce the crown openings needed for robust oak or other hardwood mast production (Cocking et al. 2012). Chinquapin is dependent on frequent disturbance to retain a competitive foothold in the forest (OWIC II 2016). Animal species in this forest type are also fire dependent. Black-tailed deer depends on a mosaic of burned and unburned habitat (Innes 2013), and pacific fisher benefit from burned landscapes for tribal hunting and foraging (Hanson 2013, Long et al. 2016).
Vulnerabilities Resulting from Increasing Frequency of High Severity Fire
While this forest band is fire-dependent, stand dynamics and individual species in this forest type face vulnerabilities in light of the increasing frequency of high severity fires. A few species such as deer benefit from high severity fires, when the proportion or extent of burn severities (low to high) are diverse. Oaks are not highly fire resistant and even mature black oak trees are susceptible to topkill by fire. Black oaks may re-sprout, but it takes time for these trees to reach maturity for acorn production (Cocking et al. 2012, Stephens and Finney 2002). Fisher dens and denning habitat can be decreased when hardwoods are burned or fall down and no longer suitable habitat.
Vulnerabilities Exacerbated by Non-Tribal Management Actions
Federal fire suppression practices that have prevailed over the past century have led to declines in black oaks and other fire-dependent species (Fryer 2007). Under fire suppression coniferous saplings that would normally be eliminated by fire mature into fire resistant diameters with thick enough bark or structure where they may outgrow and shade the light-dependent species on the chinquapin forest band. During high severity fire events many fire suppression actions create further vulnerabilities to species in this forest elevation zone. Fire fighting tactic of “burning out” along the fire lines creates areas of very high severity fire (Lake pers comm as Resource Advisor). Timber fallers often intentionally cut chinquapin and black oaks considered “hazard trees’ during fire suppression activities preemptively because they may have cavities in which fire can ignite and then pose a threat to the fire line control capacity. However such cavities are important habitat for pacific fisher (Long et al. 2016). Black oaks snags are also often fallen with fire line construction activities. Deer benefit even from high severity fires, but if fires are very hot and fire fighters don’t leave any islands of green for refugia, deer may face direct mortality and significant impacts from lack of forage, or literally being burned out of animal safe sites of interior unburned portions of the larger wildfire. To further illustrate the complex of vulnerabilities high severity fire poses for Karuk species of importance in this elevation zone we provide species profiles for chinquapin (sunyíthih), black oak (xánthiip), Pacific fisher (tatkunuhpíithvar), black-tailed deer (púufich), and porcupine (kaschiip).
Chinquapin / Sunyíthih / Castanopsis chrysophylla
Black Oak / Xánthiip / Quercus kelloggii
Pacific Fisher / Tatkunuhpíithvar / Pekania pennanti
Black Tailed Deer / Púufich / Odocoileus hemionus
Porcupine / Kaschiip / Erethizon dorsatum
High Elevation Forest Vulnerabilities
High elevation forest are defined here as those existing above the chinquapin band (note however that the shrub form chinquapin may be found at these elevations -but is this non-producing?). Like their lower elevation counterparts, the high elevation forests within Karuk ancestral territory are biologically rich and incredibly species diverse. Taylor et al. (2006) note, “The conifer component of montane forests can be quite diverse and up to 17 conifer species have been identified in some watersheds in the north central Klamath Mountains” (p. 175). Karuk foods and cultural use species occurring in this forest type include the Sugar Pine, Port Orford Cedar, Incense Cedar, Green Leaf Manzanita, Saddler oak, Gooseberry, Black Cap Raspberries, Trailing Black berries, Lilies (tiger/Cascade lilies) and Beargrass (which especially occurs towards coast where has fog). Wolf (ikxâavnamich) is also important here.
Karuk Cultural burning enhances species in the high elevation forest type, making nutrients available in soils, releasing the seeds in sugar pine cones, stimulating growth and flowering of beargrass and minimizing fuel loads to protecting from high severity fires. Cultural burning at roughly 5-10 year intervals across the landscape creates multiple good gathering areas for beargrass (Hummell et al. 2012).
Vulnerabilities Resulting from Increasing Frequency of High Severity Fire
While this forest type benefits from regular low severity fire, high severity fires can damage trees and burn duff into soil deep enough to destroy bear grass rhizomes (Hummel et al. 2012). Damage to forest duff from very hot fires can delay or prevent the re-establishment of beargrass. Mature trees stressed by fire injury are susceptible to bark beetle and other insects which increases future fire severity (Fettig et al. 2013). In the longer-term aftermath of multiple high severity fires, there is risk of loss of these forest types to brush fields (Donato et al. 2009). With repeated high severity fires brush and down woody material can hinder Sugar pine reestablishment and increase risk of repeated high severity fires. Diseases such as white pine blister rust, coupled with fire exclusion also threaten the persistence of sugar pines (van Mantgem et al. 2004).
Vulnerabilities Exacerbated by Non-Tribal Management Actions
Fire fighting tactics themselves have particular negative impacts on species in the high elevation forest zone. For example Sugar Pines are intentionally cut down preemptively during fire line construction because they “could burn” since these trees form snags and fire can enter their cavities (Lake pers. comm., obs READ experience). If salvage logging takes place after fires, Sugar pines are often targeted as economically valued species (Sessions et al. 2004).
Sugar Pine/ Ússip / Pinus lambertiana
Bear Grass / Panyúrar / Xerophyllum tenax
Wet Meadow Vulnerabilities
Karuk ancestral territory contains a number of higher elevation wet meadow systems which are both critical habitat for species and important for hydrologic, ecological and fire dynamics in lower elevations (below the meadows). Wet meadows are found scattered throughout the higher elevation forest and high country. Important species occurring in wet meadows include black bear, elk and deer (summer), trailing blackberry, Mariposa and Panther lilies, Wild Turnip, and multiple kids of Indian potatoes (e.g. Brodiaea coronaria). Wet meadows not only contain many species of importance, they are important indirectly for their connection to other habitat types. Wet meadows are dependent upon snowpack from upper elevation high country, and in turn provide a steady release of water that gives protection from flooding to forested areas below.
Wet meadow systems are dependent upon ignitions from human and natural sources (Dwire and Kauffman 2003, Turner et al. 2011, Lake and Long 2014). In the absence of fire, the encroachment of conifers leads to a cycle in which the water table to drop and meadows dry up. As the soil in formerly wet meadow areas dries out, upland species that cannot have their roots saturated and therefore formerly excluded by the higher soil moisture can now thrive and enter the former wet meadow system as competitors. These drier soils are more conducive to Douglas fir, true fires (Abies spp) and other hardwood trees which were kept out before, continuing a cycle of transition away from the meadow system (Halpern et al. 2010). Numerous wet meadows within Karuk ancestral territory are being lost through this process, especially at the middle to high elevations. This same cycle of fire suppression, conifer encroachment, changing soil moisture dynamics leading to further encroachment of conifers and other species also takes place around springs, causing springs to dry up.
Vulnerabilities Resulting from Increasing Frequency of High Severity Fire
Wet meadow systems under various climatic regimes, generally are relatively protected from fires due to site moisture (soil and live vegetation). Nonetheless recent observations of fires in Karuk territory indicate expansion of fire into riparian zones where it did not previously occur. While burning is essential for the maintenance of wet meadow habitats, high severity fire has the potential to cause direct mortality to species. Historically, fire occurrence at higher elevations was controlled by biophysical parameters (slope position, aspect, soil types, topography) and the fuel loading receptive to ignition and fire spread. Tree and shrub encroachment into meadow can alter the fuel load properties in the soil and above. High severity fires which burn from the forest to meadow transition can increase the depth and persistence of higher severity more lethal fire effects to species associated with meadow habitats
Vulnerabilities Exacerbated by Non-Tribal Management Actions
Probably the main intersecting vulnerability to wet meadows comes from their severely reduced range due to fire exclusion since wet meadows are a generally threatened ecosystem type. Other climate related drivers such as changing patterns of precipitation and temperature are however likely more dominant threats to these systems than the increasing frequency of high severity fires per se.
Leopard Lily / Mahtáyiith / Lilium pardalinum ssp. Wigginsii
High Country Vulnerabilities
High country is defined as montane and into the subalpine zone where sugar pines drop out (see Taylor et al. 2006). Although the high country may have fewer species used directly for food, fiber and medicine than areas lower down, this habitat zone is nonetheless critically important in relation to the health of other parts of the ecosystem. For example healthy meadow systems in the high country provide a buffer for flooding, sustaining water throughout the summer and decreasing the potential impacts of erosion in lower elevations. The high country is key for Karuk cultural and spiritual activity Chartkoff 1983, Wylie 1976). Especially during summer, families and individuals journey from lower elevation zones to harvest and process foods, materials and medicines, to hunt, fish, and pray. Fires are set on Offield mountain (Ma’ and Sa’Tue’yee [upper and lower mountain peaks] in particular as part of World Renewal ceremonies in late summer (Kroeber and Gifford 1952). Foods, fibers and medicines of particular importance to Karuk people occurring in the high country include: kishvuuf, wild onion, beargrass, huckleberry, princess pine, Oregon grape, and sugar pine (at lower portion of this zone). Turner et al. (2011) write, “these environments and their plant resources have received little detailed attention in ethnographic literature, and their importance to Indigenous Peoples often remains unrecognized.”
Karuk people have used fire to tend this habitat zone since time immemorial. Burning in these areas often occurs along trail networks, targeting meadow areas and patches of particular food and cultural use species such as huckleberry.
Increasing Frequency of High Severity Fire: Vulnerabilities to High Country
While species in the high country have been adapted to relatively frequent low intensity fire, predicted increases in the frequency of high severity fires pose vulnerabilities to the high country. Historically, this habitat zone was protected from such fires by the presence of snowpack (Olson et al. 2012). The high country is also vulnerable in light of other climate impacts, especially extended drought and the loss of snow given trends towards greater percentage of precipitation falling as rain (Olson et al. 2012).
References
Alves, R.R. and Rosa, I.M. 2007. “Biodiversity, Traditional Medicine And Public Health: Where Do They Meet?” Journal of Ethnobiology and Ethnomedicine. 3:1.
American Indian Technical Services, Inc. [AITS]. 1982. “Anthropological Study of the Hupa, Yurok, and Karok Indian Tribes of Northwestern California.” Final Report. U.S. Department of Interior, Bureau of Indian Affairs. Report on file with BIA or Lake USFS-PSW.
Anderson, M.K., 1997. From tillage to table: The indigenous cultivation of geophytes for food in California. Journal of Ethnobiology, 17(2), pp.149-169.
Anderson, M. Kat. 2005.Tending The Wild: Native American Knowledge And The Management Of California’s Natural Resources. Berkeley, CA: University of California Press. 558 p.
Anderson, M. Kat. 2006. “The Use Of Fire By Native Americans In California.” In Sugihara, N.G.; van Wagtendonk, J.W.; Fites-Kaufman, J.; Shaffer, K.E.; Thode, A.E., eds. Fire in California’s ecosystems. Berkeley, CA: University of California Press: 417–430.
Anderson, M.Kat. 2007. “Indigenous Uses, Management, And Restoration Of Oaks Of The Far Western United States.” Tech. Note No. 2. Washington, DC: U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Center. 20 p.
Anderson, M. Kat, and Eric Wohlgemuth. 2012. “California Indian Proto-Agriculture: Its Characterization And Legacy.” Biodiversity in Agriculture: Domestication, Evolution, and Sustainability: 190-224.
Anderson, M. Kat & Lake, Frank K. 2013. “California Indian Ethnomycology And Associated Forest Management.” Journal of Ethnobiology, 33(1), 33-85.
Anderson, M. K. and F. K. Lake 2016. Common Beargrass, USDA Natural Resource Conservation Service Plant Guide [Final Draft-not available on line].
Anonymous. 1992. Fires as agents of biodiversity: pyrodiversity promotes biodiversity. Proceedings of the symposium on biodiversity of northwestern California. 150 p.
Bachelet, D., Johnson, B.R., Bridgham, S.D., Dunn, P.V., Anderson, H.E. and Rogers, B.M., 2011. Climate change impacts on western Pacific Northwest prairies and savannas. Northwest Science, 85(2), pp.411-429.
Band, Anne-Louise. 1996. “Population Characteristics And Habitat Use Of Porcupines In A Partially Burned Landscape In Northwestern Wyoming.” Theses, Dissertations, Professional Papers. University of Montana. Paper 6531. http://scholarworks.umt.edu/cgi/viewcontent.cgi?article=7566&context=etd. (July 14, 2016).
Barr, B.R., Koopman, M.E., Williams, C.D., Vynne, S., Hamilton, R., Doppelt, B. and Climate Leadership Initiative. 2010. “Preparing for climate change in the Klamath Basin.”
Beamish, R. J., Bouillon, D. R. 1993. “Pacific Salmon Production Trends In Relation To Climate.” Canadian Journal of Fisheries and Aquatic Sciences. 50:1002-1016
Bissell, H. D., Strong, Helen. 1955. “The Crude Protein Variations In The Browse Diet Of California Deer.” California Fish and Game. 41:145-155.
Biswell, H.H. 1989.Prescribed Burning In California Wildlands Vegetation Management. Univ of California Press.
Bowcutt, Frederica. 2013. “Tanoak Landscapes: Tending a Native American Nut Tree.” Madroño. 60: 64-86.
Bowcutt, Frederica. 2015.The Tanoak Tree: An Environmental History of a Pacific Coast Hardwood. University of Washington Press.
Brooks, M.L., D’antonio, C.M., Richardson, D.M., Grace, J.B., Keeley, J.E., DiTomaso, J.M., Hobbs, R.J., Pellant, M. and Pyke, D. 2004. “Effects Of Invasive Alien Plants On Fire Regimes.” BioScience. 54: 677-688.
Brown, P., Lyson, M. and Jenkins, T., 2011. “From Diagnosis To Social Diagnosis.” Social Science & Medicine, 73(6), pp.939-943.
Bull, Evelyn L.; Clark, Abe A.; Shepherd, Jay F. 2005. “Short-Term Effects Of Fuel Reduction On Pileated Woodpeckers In Northeastern Oregon—A Pilot Study.” Res. Pap. PNW-RP-564. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 17 p.
Bull, Evelyn L., Nielsen-Pincus, Nicole, Wales, Barbara C., Hayes, Jane L. 2007. “The Influence Of Disturbance Events On Pileated Woodpeckers In Northeastern Oregon.” Forest Ecology and Management. 243: 320-329.
Burton, Timothy A. 2005. “Fish And Stream Habitat Risks From Uncharacteristic Wildfire: Observations From 17 Years Of Fire-Related Disturbances On The Boise National Forest, Idaho.” Forest Ecology and Management 211: 140-149.
Bury, R. Bruce; Major, Donald J.; Pilliod, David. 2002. “Responses Of Amphibians To Fire Disturbance In Pacific Northwest Forests: A Review.” In: Ford, W. Mark; Russell, Kevin R.; Moorman, Christopher E., eds. Proceedings: the role of fire for nongame wildlife management and community restoration: traditional uses and new directions. Gen. Tech. Rep. NE-288. Newtown Square, PA: U.S. Dept. of Agriculture, Forest Service, Northeastern Research Station: 34-42. http://www.nrs.fs.fed.us/pubs/gtr/gtr_ne288/gtr_ne288_034.pdf. (July 12, 2016).
California Native Plant Society [CNPS]. 2016. Tiger Lily: Lilium Pardalium. http://calscape.cnps.org/Lilium-pardalinum-(Tiger-Lily)?srchcr=sc560cf817b7059. (August 9, 2016).
Californiaherps.com. 2016. California Giant Salamander – Dicamptodon ensatus. http://www.californiaherps.com/salamanders/pages/d.ensatus.html. (July 12, 2016).
Californiaherps.com I. 2016. Santa Cruz Gartersanke – Thamnophis atratus atratus. http://www.californiaherps.com/snakes/pages/t.a.atratus.html. (August 11, 2016).
Cardiff, E.A. 1993. “Desert Riparian-Freshwater Marsh [Breeding bird census]. Resident bird counts 1992.” J. Field Ornithol. 64 (suppl.):92-93.
Cardiff, E.A. 1996. “Desert Riparian-Freshwater Marsh [Breeding bird census]. Resident bird counts 1995.” J. Field Ornithol. 67 (suppl.):75.
Chartkoff, Joseph L. and Kerry K. Chartkoff. 1975. “Late Period Settlement of the Middle Klamath River of Northwest California.” American Antiquity. 40: 172-179
Chartkoff, Joseph L. 1983. A Rock Feature Complex from Northwestern California. American Antiquity, Vol. 48, No. 4, pp. 745-760. Published by Society for American Archaeology
Cocking, Matthew I., Varner, J. Morgan, Sherriff, Rosemary L. 2012. “California Black Oak Responses To Fire Severity And Native Conifer Encroachment In The Klamath Mountains.” Forest Ecology and Management 270: 25-34.
Collen, P.; Gibson, R.J. 2001. “The General Ecology of Beavers (Castro spp.), as Related to their Influence on Stream Ecosystems and Riparian Habitats, and the Subsequent Effects on Fish—a Review.” Reviews in Fish Biology and Fisheries. 10: 439-461.
Columbia Basin Bulletin [CBB]. 2015. Study: Contaminants Found in Pacific Lamprey Could Be Contriuting to Population Decline. http://www.cbbulletin.com/433727.aspx. (July 26, 2016)
Crane, M. F. 1990. Xerophyllum tenax. In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: http://www.fs.fed.us/database/feis/. (2016, July 28).
Cuerrier, A., Turner, N.J., Gomes, T.C., Garibaldi, A. and Downing, A. 2015. “Cultural Keystone Places: Conservation and Restoration in Cultural Landscapes.” Journal of Ethnobiology. 35: 427-448.
Dale, V.H., Joyce, L.A., McNulty, S., Neilson, R.P., Ayres, M.P., Flannigan, M.D., Hanson, P.J., Irland, L.C., Lugo, A.E., Peterson, C.J. and Simberloff, D. 2001. “Climate Change And Forest Disturbances: Climate Change Can Affect Forests By Altering The Frequency, Intensity, Duration, And Timing Of Fire, Drought, Introduced Species, Insect And Pathogen Outbreaks, Hurricanes, Windstorms, Ice Storms, Or Landslides.” BioScience. 51:723-734.
Dasmann, Raymond F., Dasmann, William P. 1963. “Mule Deer In Relation to a Climate Gradient.” The Journal of Wildlife Management. 27: 196-202.
Davis, F.W., Seo, C. and Zielinski, W.J., 2007. Regional Variation In Home‐Range‐Scale Habitat Models For Fisher (Martes Pennanti) In California. Ecological Applications, 17(8), pp.2195-2213.
DeBano, L.F., Neary, D.G. and Ffolliott, P.F., 1998. Fire effects on ecosystems. John Wiley & Sons.
Dietrich, Joseph P.; Myers, Mark S.; Strickland, Stacy A.; Van Gaest, Anha; Arkoosh, Mary R. 2012. “Toxicity Of Forest Fire Retardant Chemicals To Stream-Type Chinook Salmon Undergoing Parr-Smolt Transformation.” Environmental Toxicology and Chemistry. (32) 236-247.
Driver, Harold E. 1939. “Culture Element Distributions: X Northwest California.” Anthropological Records. 1: 297-433.
Dunham, J.B., Young, M.K., Gresswell, R.E. and Rieman, B.E. 2003. Effects of fire on fish populations: landscape perspectives on persistence of native fishes and nonnative fish invasions. Forest Ecology and Management, 178(1), pp.183-196.
Dunn, J.L.; Garrett, K.L.. 1997.A Field Guide To Warblers Of North America. Houghton Mifflin, Boston, Massachusetts.
Dwire, K.A. and Kauffman, J.B., 2003. Fire and riparian ecosystems in landscapes of the western USA. Forest Ecology and Management, 178(1), pp.61-74.
Eriksen, Christine, Hankins, Don L. 2014. “The Retention, Revival, And Subjugation Of Indigenous Fire Knowledge Through Agency Fire Fighting In Eastern Australia And California.” Society & Natural Resources 27: 1288-1303.
Farris, Kerry L., Zack, Steve. 2005. “Woodpeck-snag interactions: an overview of current knowledge in ponderosa pine systems.” USDA Forest Service Gen. Tech. Rep. PSW-GTR-198. Pages: 183-195
Fettig, C.J., Reid, M.L., Bentz, B.J., Sevanto, S., Spittlehouse, D.L. and Wang, T., 2013. Changing climates, changing forests: A western North American perspective. Journal of Forestry, 111(3), pp.214-228.
Fite-Kaufman, JoAnne. 2006. Fire Behavior Team Assessment Report for the Orleans Complex-Somes Fire. On file with Karuk Tribe.
Fleischhacker, S., Byrd, R.R., Ramachandran, G., Vu, M., Ries, A., Bell, R.A. and Evenson, K.R. 2012. “Tools For Healthy Tribes: Improving Access To Healthy Foods In Indian Country.” American Journal Of Preventive Medicine. 43: S123-S129.
Flitcroft, R.L., Falke, J.A., Reeves, G.H., Hessburg, P.F., McNyset, K.M. and Benda, L.E., 2016. Wildfire may increase habitat quality for spring Chinook salmon in the Wenatchee River subbasin, WA, USA. Forest Ecology and Management, 359, pp.126-140.
Fryer, Janet L. 2007. “Quercus kelloggii.” In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: http://www.fs.fed.us/database/feis/. [2016, August 2].
Garibaldi, A., Turner, N. 2004. “Cultural keystone species: implications for ecological conservation and restoration.” Ecology and Society. 9:1.
Gifford, Edward W. 1939. “Karok Field Notes (Ethnological Document No. 174).” Department and Museum of Anthropology, University of California Bancroft Library, Berkeley, CA.
Golightly, Richard T.; Penland, Talitha F.; Zielinski, William J.; Higley, Mark. 2006. “Fisher Diet in the Klamath/ North Coast Bioregion.” Unpublished report, Department of Wildlife, Humboldt State University, Arcata, California.
Grifantini, M.C., Stuart, J.D. and Fox, L., 1992. Deer habitat changes following wildfire, salvage logging and reforestation, Klamath Mountains, California. In Proceedings of the symposium on biodiversity of northwestern California, Report (Vol. 29, pp. 163-167).
Habeck, R. J. 1992. Pinus lambertiana. In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service,
Rocky Mountain Research Station, Fire Sciences Laboratory (Producer).
Available: http://www.fs.fed.us/database/feis/. (2016, July 20)
Halpern, A. 2016. “Prescribed fire and tanoak (Notholithocarpus densiflorus) associated cultural plant resources of the Karuk and Yurok Peoples of California.” PhD diss., University of California, Berkeley.
Halpern, C.B., Antos, J.A., Rice, J.M., Haugo, R.D. and Lang, N.L., 2010. Tree invasion of a montane meadow complex: temporal trends, spatial patterns, and biotic interactions. Journal of Vegetation Science, 21(4), pp.717-732.
Hamlet, A.F. 2011. “Assessing Water Resources Adaptive Capacity To Climate Change Impacts In The Pacific Northwest Region Of North America.” Hydrology and Earth System Sciences. 15: 1427-1443.
Hanson, Chad T. 2013. “Habitat Use of Pacific Fishers in a Heterogeneous Post-Fire and Unburned Forest Landscape on the Kern Plateau, Sierra Nevada, California.” The Open Forest Science Journal. 6: 24-30.
Harrington, John Peabody. 1932.Tobacco among the Karuk Indians of California. Bulletin (Smithsonian Institution. Bureau of American Ethnology); 94. Washington: U.S. Govt. Print. Off.
Hartwig, C.L., Eastman, D.S., and Harestad, A.S. 2004. Characteristics of pileated woodpecker (Dryocopus pileatus) cavity trees and their patches on southeastern Vancouver Island, British Columbia, Canada. Forest Ecology and Management 187: 225-234.
Hillman, Leaf, Salter, John F. 1997. “Environmental Management: American Indian Knowledge And The Problem Of Sustainability.” Forests, Trees and People Newsletter (FAO)(Sweden)
Hillman, Lisa R. 2016. “Karuk Tribe in the Era of Climate Change.” Conference Paper prepared for the 2016 Klamath Basin Tribal Youth Exchange, Karuk Tribe.
Hitt, Nathaniel P. 2003. “Immediate Effects Of Wildfire On Stream Temperature.” 171-173.
Holsinger, L., Keane, Robert E., Isaak, Daniel J., Eby, Lisa, Young, Michael K. 2014. “Relative Effects of Climate Change and Wildfires on Stream Temperatures: A Simulation Modeling Approach in a Rocky Mountain Watershed.” Climatic Change. 124: 191-206.
Hood, Glynnis A.; Bayley, Suzzane E.; Olson, Wes. 2007. “Effects of Prescribed Fire on Habitat of Beaver ( Castor Canadensis) in Elk Island National Park, Canada.” Forest Ecology and Management. 239: 200-09.
Hosford, David, Pilz, David, Molina, Randy, Amaranthus, Michael.1997. “Ecology and Management of the Commercially Harvested American Matsutake Mushroom.” General Technical Report PNW-GTR-412. USDA Forest Service Pacific Northwest Research Station, Portland.
Hosten, P.E., Hickman, O.E., Lake, F.K., Lang, F.A., Vesely, D. 2005. “Oak Woodlands And Savannas.” In Higgs, E.; Apostol, D.; Sinclair, M., eds. Restoring the Pacific Northwest: The Art and Science of Ecological Restoration in Cascadia. Washington DC: Island Press: 63-96.
Hummel, S., Foltz-Jordon, S., Polasky, S. 2012. “Natural and cultural history of beargrass (Xerophyllum tenax).” USDA For. Serv., Gen. Tech. Rep. PNW-GTR-864, Pacific Northwest Research Station, Portland, OR. 80 p.
Hummel, S., Lake, F. K. 2015. “Forest Site Classification for Cultural Plant Harvest by Tribal Weavers Can inform Management.” Journal of Forestry. 113: 30-39.
Hummel, S., Lake, F.K., Watts, A. 2015. “Using Forest Knowledge: How Silviculture Can Benefit From Ecological Knowledge Systems About Beargrass Harvesting Sites.” USDA Forest Service, PNW-GTR-912.
Isaak, Daniel J., Charles H. Luce, Bruce E. Rieman, David E. Nagel, Erin E. Peterson, Dona L. Horan, Sharon Parkes, and Gwynne L. Chandler. 2010. “Effects of Climate Change and Wildfire on Stream Temperatures and Salmonid Thermal Habitat in a Mountain River Network.” Ecological Applications. 20: 1350-1371.
Innes, Robin J. 2011. “Cervus elaphus.” In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: http://www.fs.fed.us/database/feis/. [2016, July 15]
Innes, Robin J. 2013. “Odocoileus hemionus.” In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: http://www.fs.fed.us/database/feis/animals/mammal/odhe/all.html. [2016, July 18]
Karr JR, Rhodes JJ, Minshall GW, et al. 2004. The effects of postfire salvage logging on aquatic ecosystems in the American west. BioScience 54: 1029–33.
Karuk Tribe Department of Natural Resources [Karuk DNR]. 2010. “Karuk Tribe Draft Eco-Cultural Resources Management Plan.” http://www.karuk.us/images/docs/dnr/ECRMP_6-15-10_doc.pdf. (September 2, 2016).
Kauffman, J.B.; Martin, R.E. 1987. “Effects of fire and fire suppression on mortality and mode of reproduction of California black oak (Quercus kelloggii Newb.).” In: Plumb, T.R.; Pillsbury, M.N., eds. Proceedings of the symposium on multiple-use management of California hardwood resources. Gen. Tech. Rep. PSW-100. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: 122–126.
Kimmerer, R.W., Lake, F.K. 2001. “The Role Of Indigenous Burning In Land
Management.” Journal of Forestry. (99): 36–41.
Kremsatelr, L., Bunnellf, L. 1992. “Testing Responses To Forest Edges: The Example Of Black-Tailed Deer.” Canadian Journal of Zoology. 70: 2426-2435.
Kroeber, A.L. and Barrett, S.A. 1960.Fishing Among The Indians Of Northwestern California (Vol. 21, No. 1). Berkeley: University of California Press.
Lake, Frank. 2007. “Traditional Ecological Knowledge to Develop and Maintain Fire Regimes inNorthwestern California, Klamath-Siskiyou Bioregion: Management and Restoration of Culturally Significant Habitats.” PhD diss., Oregon State University.
Lake, Frank. 2015. “Risk Matrix-Evaluation: Species Profiles for Thin-leaf (Vaccinium membranaceum) and Evergreen (V. ovatum) in the Pacific West.” Contribution to the National Non-Timber Forest Products and Climate Change Assessment.
Lake, Frank K., Long, Jonathan W. 2014. “Fire And Tribal Cultural Resources”
Science Synthesis to Support Socioecological Resilience in the Sierra Nevada and Southern Cascade Range GENERAL TECHNICAL REPORT PSW-GTR-247 pp 173-186 http://www.treesearch.fs.fed.us/pubs/46960
Landis, T.D. 2000. “Where There’s Smoke… There’s Germination.” Native Plants Journal. 1: 25–29.
Lewis, Henry T. 1973/1993.Patterns of Indian Burning in California: Ecology and Ethnohistory in Before the Wilderness. Ballena Press.
Lininger, Jay C. 2004. “Fire History and Need for Fuel Management in Mixed Douglas-fir Forests of the Klamath-Siskiyou Region, Northwest California and Southwest Oregon, USA.” In: 2nd international wildland fire ecology and fire management congress: Proceedings; 2003 November 19; Orlando, FL. Session 4C – Fire History/Fire Regimes. [Publication location unknown]: [Publisher unknown]: 18 p. [64193]
Long, R.A., Rachlow, J.L., Kie, J.G., and Vavra, M. 2008. Fuels Reduction in a Western Coniferous Forest: Effects on Quantity and Quality of Forage for Elk. Rangeland Ecol. Manage 61: 302-313.
Long, Jonathan W., M. Kat Anderson, Lenya Quinn-Davidson, Ron W. Goode, Frank K. Lake, and Carl N. Skinner. 2016. “Restoring California Black Oak Ecosystems to Promote Tribal Values and Wildlife. Restoring California black oak ecosystems to promote tribal values and wildlife.” Gen. Tech. Rep. PSWGTR- 252. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station. 110 p.
Luce, Charles, and Rocky Mountain Research Station, Issuing Body. 2012.Climate Change, Forests, Fire, Water, and Fish : Building Resilient Landscapes, Streams, and Managers. General Technical Report RMRS ; GTR-290. Fort Collins, CO: United States Department of Agriculture, Forest Service, Rocky Mountain Research Station.
Lynn, K.; Daigle, J.; Hoffman, J.; Lake, F.; Michelle, N.; Ranco, D.; Viles, C.; Voggesser, G.; Williams, P. 2013. “The Impacts Of Climate Change On Tribal Traditional Foods.” Climatic Change (special issue). http://link.springer.com/article/10.1007/s10584-013-0736-1. (September 23, 2016).
Martinez, D. 1995. “Karuk Tribal Module of Mainstem Salmon Watershed Analysis: Karuk Ancestral Lands and People as Reference Ecosystem (or Eco-Cultural Restoration in Collaborative Ecosystem Management.” Unpublished report. On file with the U.S. Deparh11ent of Agriculture, Forest Service, Klamath National Forest, CA
McDonald, P.M. 1990. “Quercus kelloggii Newb. California black oak.” In: Burns, R.M.; Honkala, B.H., eds. Silvics of North America. Volume 2, hardwoods. Washington, DC: U.S. Department of Agriculture: 661–671.
McDonald, P.M. and N.R. Vaughn 2007. Growth of Thinned and Unthinned Hardwood Stands on a Good Site in Northern California. USDA Forest Service, PSW General Tech. Report PSW-GTR-204.
Miller, J. 2012. “Lamprey “Eels” In The Greater Northwest: A Survey Of Tribal Sources, Experiences, And Sciences.” J. Northwest Anthropol, 46(6).
Mote, P.W., Parson, E.A., Hamlet, A.F., Keeton, W.S., Lettenmaier, D., Mantua, N., Miles, E. L., Peterson, D.W., Peterson, D.L., Slaughter, R. 2003. “Preparing For Climatic Change: The Water, Salmon, And Forests Of The Pacific Northwest.” Climatic Change. 61:45-88.
Myers, Stephen. n.d. Yellow-Breasted Chat – Icteria vivens.http://www.blm.gov/ca/pdfs/cdd_pdfs/Ybch1.pdf. (August 11, 2016)
Neary, D.G., Klopatek, C.C., DeBano, L.F. and Ffolliott, P.F., 1999. Fire effects on belowground sustainability: a review and synthesis. Forest ecology and management, 122(1), pp.51-71.
Norgaard, Kari M. 2005. “The Effects of Altered Diet on the Health of the Karuk People: a Preliminary Report.” http://pages.uoregon.edu/norgaard/pdf/Effects-Altered-Diet-Karuk-Norgaard-2005.pdf. (July 18, 2016)
North, M., Innes, J. and Zald, H., 2007. “Comparison Of Thinning And Prescribed Fire Restoration Treatments To Sierran Mixed-Conifer Historic Conditions.” Canadian Journal of Forest Research, 37: 331-342.
Noss, R.F., Franklin, J.F., Baker, W.L., Schoennagel, T. and Moyle, P.B. 2006. Managing fire‐prone forests in the western United States. Frontiers in Ecology and the Environment, 4(9), pp.481-487.
Odion, D.C., Frost, E.J., Strittholt, J.R., Jiang, H., Dellasala, D.A. and Moritz, M.A. 2004. “Patterns of fire severity and forest conditions in the western Klamath Mountains, California.” Conservation Biology. 18: 927-936.
Odion, D.C., Moritz, M.A. DellaSala, D.A. 2010. “Alternative Community States Maintained By Fire In The Klamath Mountains, USA.” Journal of Ecology.98: 96-105.
Ortiz, Beverly R. 2008. “Contemporary California Indians, Oaks And Sudden Oak Death (Phytophthora ramorum).”
Ortiz, Beverly R. 2008. “Contemporary California Indian uses for food of species affected by Phytophthora ramorum.” Proceedings of the Sudden Oak Death Third Science Symposium, March 5–9, 2007, Santa Rosa, California.
OSU Oregon Wood Innovation Center [OWIC]. 2016. “Tanoak (Lithocarpus densiflorus).” http://owic.oregonstate.edu/tanoak-lithocarpus-densiflorus. (July 11, 2016).
OSU Oregon Wood Innovation Center [OWIC II]. 2016. “Giant Chinkapin (Castanopsis chrysophylla).” http://owic.oregonstate.edu/giant-chinkapin-castanopsis-chrysophylla. (August 2, 2016).
Pacific Forest Trust. 2016. Wet Meadow Habitat. https://www.pacificforest.org/habitat/wet-meadow/. (August 9, 2016).
Patton, David R.; Gordon, Janet. 1995. Fire, habitats, and wildlife. Final report. Flagstaff, AZ: U.S. Department of Agriculture, Forest Service, Coconino National Forest. 85 p. Unpublished report on file with: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT.
Peters, Josephine; Ortiz, Beverly. 2016.After the First Full Moon in April: a Sourcebook of Herbal Medicine from a California Elder. Routledge, New York, NY.
Peterson, Robin 2006. “The Role of Traditional Ecological Knowledge in Understanding a Species and River System at Risk: Pacific Lamprey in the Lower Klamath Basin.” Masters of Arts in Applied Anthropology (Thesis), Oregon State University, 167 p.
Petersen Lewis, R.S., 2009. “Yurok And Karuk Traditional Ecological Knowledge: Insights Into Pacific Lamprey Populations Of The Lower Klamath Basin.” In: Biology, Management, And Conservation Of Lampreys In North America. American Fisheries Society, Symposium. 72: 1-39.
Pettit, N.E. and Naiman, R.J. 2007. Fire in the riparian zone: characteristics and ecological consequences. Ecosystems, 10(5), pp.673-687.
Pilliod, David S.; Bury, R. Bruce; Hyde, Erin J.; Pearl, Christopher A.; Corn, Paul Stephen. 2003. “Fire and Amphibians in North America.” Forest Ecology and Management. 178: 163-181. http://leopold.wilderness.net/pubs/474.pdf. (July 16, 2016)
Pullen, R.J., 1996. Overview of the environment of native inhabitants of southwestern Oregon, late prehistoric era. US Bureau of Land Management, Medford District Office.
Rentz, E. 2003. Effects of fire on plant anatomical structure in native Californian basketry materials. San Francisco, CA: San Francisco State University. 99 p. M.S thesis
Richards, Rebecca T. 1997. “What the Natives Know: Wild Mushrooms and Forest Health.” Journal of Forestry. 95:4–10.
Richards, Rebecca T., Creasy, Max. 1996. “Ethnic Diversity, Resource Values, and Ecosystem Management: Matsutake Mushroom Harvesting in the Klamath Bioregion.” Society & Natural Resources. 9:359–374.
Rieman, Bruce, Lee, Danny, Burns, Dave, Gresswell, Robert, Young, Michael, Stowell, Rick, Rinne, John, Howell, Philip. 2003. “Status Of Native Fishes In The Western United States And Issues For Fire And Fuels Management.” Forest Ecology and Management. 178: 197-211.
Risling Baldy, C. 2013. “Why We Gather: Traditional Gathering In Native Northwest California And The Future Of Bio-Cultural Sovereignty.” Ecological Processes. 2:17
Robock, A., 1988. “Enhancement Of Surface Cooling Due To Forest Fire Smoke.” Science. 242: 911-913.
Robock, A., 1991. “Surface cooling due to forest fire smoke.” Journal of Geophysical Research. 96: 20869.
Sachro, L.L., Strong, W.L., and Gates, C.C. 2005. “Prescribed Burning Effects On Summer Elk Forage Availability In The Subalpine Zone, Banff National Park, Canada.” Journal of Environmental Management. 77: 183-193.
Salmon River Restoration Council [SRRC]. 2015. “Spring Chinook Refugia: Conservation of California’s Imperiled Wild Run.” http://srrc.org/publications/newsletters/SRRC Fall 2015 Newsletter.pdf. (July 16, 2016)
Salter, John. 2003. “White Paper On Behalf Of The Karuk Tribe Of California: A Context Statement Concerning The Effect of the Klamath Hydroelectric Project on Traditional Resource Uses and Cultural Patterns of the Karuk People Within the Klamath River Corridor.” http://mkwc.org/old/publications/fisheries/Karuk White Paper.pdf. (August 11, 2016)
Sawyer, J., O. 2007. Forests of Northwestern California. In Barbour, M. G., T. Keeler-Wolf, and A. Schoenherr A., editors. Terrestrial Vegetation of California, .
Senos, R., Lake, F.K., Turner, N., and Martinez, D. 2006. “Traditional Ecological
Knowledge and Restoration Practice.” In Restoring the Pacific Northwest: The Art and Science of Ecological Restoration in Cascadia. Apostol, D. and Sinclair, M. (eds.).
Island Press, Washington D.C. Pages: 393-426.
Sessions, J., Bettinger, P., Buckman, R., Newton, M. and Hamann, J., 2004. Hastening the return of complex forests following fire: the consequences of delay. Journal of Forestry, 102(3), pp.38-45.
Shatford, J.P.A., Hibbs, D.E., Puettmann, K.J. 2007. “Conifer Regeneration After Forest Fire In The Klamath-Siskiyous: How Much, How Soon?” Journal of Forestry. 105: 139-146.
Shebitz, D. 2005. Weaving traditional ecological knowledge into the restoration of basketry plants. Journal of Ecological anthropology 9:51-68.
Shebitz, D. J., S. H. Reichard, and P. W. Dunwiddie. 2009a. Ecological and cultural significance of burning beargrass habitat on the Olympic Peninsula, Washington. Ecological Restoration 27:306-319.
Shebitz, D.J.; Ewing, K.; Gutierrez, J. 2009b. Preliminary observations of using smokewater to increase low-elevation beargrass (Xerophyllum tenax) germination. Native Plants 10(1): 13–19.
Schenck, Sara Moffatt; Gifford, Edward Winslow. 1952. “Karok Ethnobotany.” Anthropological Records ; v. 13, No. 6. Berkeley]: [University of California Press].
Skinner, Carl N.; Taylor, Alan H.; Agee, James K. 2006. “Klamath Mountains bioregion.” In Fire in California’s Ecosystems, N. G. Sugihara, J. W. van Wagtendonk, J. Fites-Kaufmann, K. E. Shaffer, and A. E. Thode, Eds. University of California Press, Berkeley. pp. 170-194.
Skinner, C.N. 1995. “Change In Spatial Characteristics Of Forest Openings In The Klamath Mountains Of Northwestern California, USA.” Landscape Ecology. 10: 219-228.
Slauson, Keith, Zielinski, Bill. 2016. “A Science Synthesis Of Fuels And Fire Treatment On The Fisher In Support Of The Western Klamath Restoration Partnership.” Copy on file with Karuk Tribe and USFS-PSW.
Snyder, S. A. 1991. “Canis lupus.” In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station,
Fire Sciences Laboratory (Producer). Available:
http://www.fs.fed.us/database/feis/animals/mammal/calu/all.html.(2016, August 10).
Soto, Toz. 2016. Soto Karuk Fisheries Program Director, In-person communication.
Stein, S.J., Price, P.W., Abrahamson, W.G., Sacchi, C.F. 1992. “The effect of fire on stimulating willow regrowth and subsequent attack by grasshoppers and elk.” Oikos. 65: 190-196.
Stephens, S. L., and L. W. Ruth. 2005. Federal forest-fire policy in the United States. Ecological Applications 15:532-542.
Stone, E. C. 1951. “Notes And Comment: The Stimulative Effect Of Fire On The Flowering Of The Golden Brodiaea (Brodiaea ixioideswats. Var. Lugens Jeps.).” Ecology. 32:534–37.
Strange, Joshua S. 2010. “Upper Thermal Limits to Migration in Adult Chinook Salmon: Evidence from the Klamath River Basin.” Transactions of the American Fisheries Society. 139:4, 1091-1108, DOI: 10.1577/T09-171.1
Streif, B. 2008. “Fact Sheet: Pacific Lamprey.” US Fish and Wildlife Service, Portland, OR. https://www.fws.gov/oregonfwo/species/data/pacificlamprey/documents/012808pl-factsheet.pdf. (July 26, 2016).
Stuart, J.D. and Salazar, L.A., 2000. Fire history of white fir forests in the coastal mountains of northwestern California. Northwest Science 74(4): 280-285
Sugihara, N. G. 2006. Fire in California’s Ecosystems. University of California Pr, .
Sweitzer, Rick A. 2012. “Porcupines An Increasingly Rare Sight in California Mid‐elevation Mixed Conifer Forests: Consequences for Conservation of Pacific Fishers.” Presentation. http://caforestpestcouncil.org/wp-content/uploads/2013/01/rick-swietzer.pdf. (July 20, 2016)
Swezey, S. L. and R.F. Heizer. 1977. “Ritual Management of Salmonid Fish Resources in California.” Journal of Cal. Anthropology. 4: 7-29.
Taruscio, T.G., Barney, D.L. and Exon, J., 2004. Content and profile of flavanoid and phenolic acid compounds in conjunction with the antioxidant capacity for a variety of northwest Vaccinium berries. Journal of agricultural and food chemistry, 52(10), pp.3169-3176.
Taylor, Alan H., Skinner, Carl N. 2003. “Spatial Patterns And Controls On Historical Fire Regimes And Forest Structure In The Klamath Mountains.” Ecological Applications 13: 704-719.
Tesky, Julie L. 1993. “Castor canadensis.” In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer).
Available: http://www.fs.fed.us/database/feis/. (2016, August 8).
Thompson, J.R. and Spies, T.A., 2010. Factors associated with crown damage following recurring mixed-severity wildfires and post-fire management in southwestern Oregon. Landscape Ecology, 25(5), pp.775-789.
Turner, N.J.; Deur, D.; Mellot, C.R. 2011. ““Up On The Mountain:” Ethnobotanical
Importance Of Montane Sites In Pacific Coastal North America.” Journal of
Ethnobiology. 31: 4–43.
US Forest Service Pacific Northwest Research Station [USFS PNRS]. 2003. “Coming Home to Roost: the Pileated Woodpecker as Ecosystem Engineer.” Science Findings. Issue 57. http://www.fs.fed.us/pnw/sciencef/scifi57.pdf. (July 19, 2016).
Van Mantgem, P.J., Stephenson, N.L., Keifer, M. and Keeley, J., 2004. Effects of an introduced pathogen and fire exclusion on the demography of sugar pine. Ecological Applications, 14(5), pp.1590-1602.
Vance, N., M. Borsting, D. Pilz, and J. Freed. 2001. “Special Forest Products.” Species Information Guide For The Pacific Northwest. USDA Forest Service, Pacific Northwest Research Station, PNW-GTR-513, Portland, OR.
Viess, Debbie. 2016.Tricholoma magnivelare: The Marvelous Matsutake. Bay Area Mycological Society. http://www.bayareamushrooms.org/mushroommonth/matsutake.html. (July 28, 2016).
Wondzell, S.M. and King, J.G., 2003. “Postfire erosional processes in the Pacific Northwest and Rocky Mountain regions.” Forest Ecology and Management. 178: 75-87.
Wylie, H.G. 1976. Tsektsels: A Disscussion of Payer Seats in Northwestern California. Report on file with USDA Forest Service, Six Rivers National Forest. Eureka, Ca.
Yocom, Charles F. 1971. “Invasion of Humboldt and Del Norte Counties of Northwestern California by Porcupines.” The Murrelet. 52: 1-6.
Zielinski, William J., Dunk, Jeffrey R., Yaeger, J. Scott, LaPlante, David W. 2010. “Developing And Testing A Landscape-Scale Habitat Suitability Model For Fisher (Martes Pennati) In Forest Of Interior Northern California.” Forest Ecology and Management. 260: 1579-1591.
Copyright © 2016 the Karuk Tribe. All rights reserved.
Unless otherwise indicated, all materials on these pages are copyrighted by the Karuk Tribe. All rights reserved. No part of these pages, either text or image may be used for any purpose other than personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.